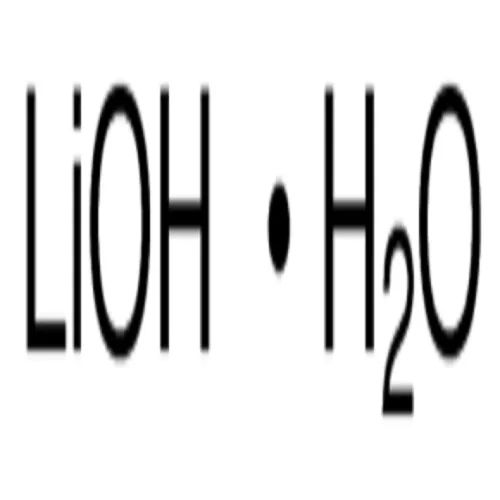LITHIUM HYDROXIDE MONOHYDRATE
3810 INR/Kilograms
Product Details:
- Purity 99.995%
- Density 1.46 Gram per cubic centimeter(g/cm3)
- Molecular Formula LIOH.H2O
- Boiling point 924 Deg C
- Molecular Weight 41.96 Grams (g)
- Storage Room Temperature
- CAS No 1310-66-3
- Click to View more
X
LITHIUM HYDROXIDE MONOHYDRATE Price And Quantity
- 25 Kilograms
- 3810 INR/Kilograms
LITHIUM HYDROXIDE MONOHYDRATE Product Specifications
- Room Temperature
- Industrial
- Industrial Grade
- 924 Deg C
- 1.46 Gram per cubic centimeter(g/cm3)
- LIOH.H2O
- 1310-66-3
- 41.96 Grams (g)
- LITHIUM HYDROXIDE MONOHYDRATE
- 99.995%
LITHIUM HYDROXIDE MONOHYDRATE Trade Information
- Cheque
- All India
Product Description
Lithium Hydroxide Monohydrate is an industrial grade chemical with a molecular weight of 41.96 grams. With a purity of 99.995%, it is commonly used in various industrial applications. Its molecular formula is LIOH.H2O and its boiling point is 924 degrees Celsius. It has a density of 1.46 grams per cubic centimeter and can be stored at room temperature. This chemical is widely used in the production of lithium-ion batteries, lubricating greases, and air conditioning systems. It is also used as a catalyst in organic reactions. Lithium Hydroxide Monohydrate is an essential component in the manufacturing of ceramics and glass. Its high purity makes it suitable for use in electronic applications.
FAQs of LITHIUM HYDROXIDE MONOHYDRATE:
Q: What is the purity of Lithium Hydroxide Monohydrate?
A: Lithium Hydroxide Monohydrate has a purity of 99.995%.Q: What is the molecular weight of Lithium Hydroxide Monohydrate?
A: The molecular weight of Lithium Hydroxide Monohydrate is 41.96 grams.Q: What is the application of Lithium Hydroxide Monohydrate?
A: Lithium Hydroxide Monohydrate is mainly used in industrial applications such as the production of lithium-ion batteries, lubricating greases, and air conditioning systems.Q: What is the storage condition for Lithium Hydroxide Monohydrate?
A: Lithium Hydroxide Monohydrate can be stored at room temperature.Q: What is the molecular formula of Lithium Hydroxide Monohydrate?
A: The molecular formula of Lithium Hydroxide Monohydrate is LIOH.H2O.Enter Buying Requirement Details